Pollution, allergies, and upper respiratory infections caused by viruses such as rhinovirus and coronaviruses like COVID-19* are growing concerns worldwide. Our HOWARU® probiotic solutions that support overall immune health and bolster healthy respiratory function in adults meet our HOWARU® VERIFY protocol.
*Taking a probiotic does not decrease the risk of getting COVID-19 and is not intended to diagnose, treat, cure, or prevent any disease.
Study aim
To determine if daily intake of a formulation containing Bifidobacterium lactis Bl-04® would have a positive impact in the prevention of upper respiratory tract illness (URTI) episodes.
Study design
5-month, double-blind, placebo controlled
Study method
310 healthy, physically active adults randomized to two treatment groups*:
- Placebo
- Bl-04® (2 billion CFU)
*An additional probiotic formula was investigated in this study, but results are not reported here.
Evaluation of symptoms and physical activity were conducted via Web-based questionnaire. The signs and symptoms of URTI included a scratchy throat, sore throat, sneezing, stuffy nose, or runny nose. A diagnosis of URTI was made when two or more symptoms were recorded for three or more consecutive days.
Study results
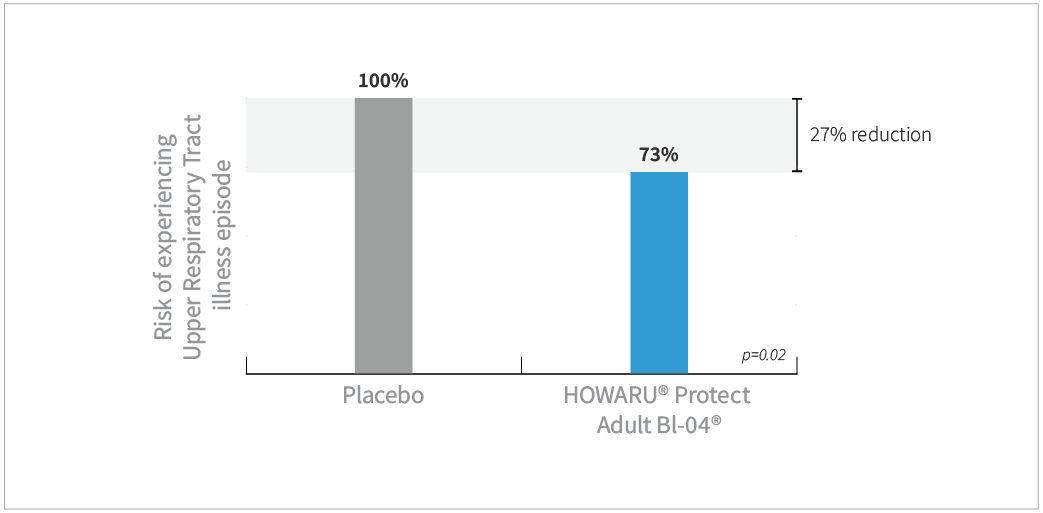
- Subjects taking Bl-04® had 27% less risk of experiencing URTI episodes compared to placebo (P=0.02)
Study aim
To study the effect of B. lactis Bl-04® on innate and adaptive immune responses, and nasal rhinovirus load for 5 days following a rhinovirus challenge infection.
Study design
Randomized, double-blind, placebo controlled
Study method
115 healthy adults were randomized to two treatment groups:
- Placebo
- Bl-04® (2 billion CFU)
- All participants challenged with nasal application of rhinovirus
Markers of immune function (interleukin 8 and other cytokines) and rhinovirus levels were measured from nasal washes as well as other endpoints.
Study results
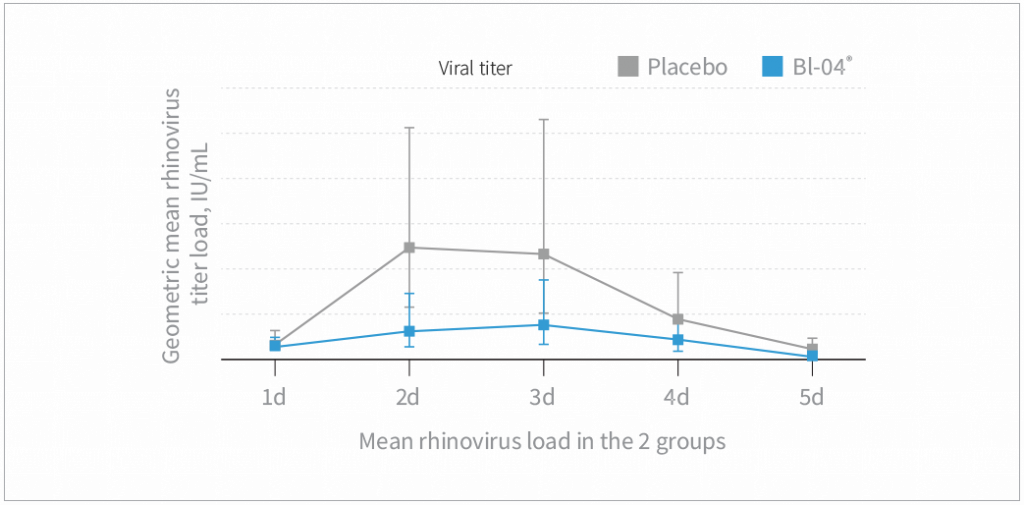
- Bl-04® supplementation decreased the amount of rhinovirus in nasal washes during the 5 days following a rhinovirus challenge compared to placebo (P=0.026)
REFERENCES:
1. West NP, Horn PL, Pyne DB, et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014;33(4):581-587. 2. Turner RB, Woodfolk JA, Borish L, et al. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection – a randomized controlled trial. Benef Microbes. 2017;8(2):207-215.
